Explain the Difference Between Exothermic and Endothermic Reactions
In contrast exothermic systems give up heat or light energy as the reaction proceeds. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of.

Exothermic And Endothermic Reactions Definition Examples And Differences
When a chemical reaction occurs energy is transferred to or from the surroundings.

. Energy is negative in exothermal reactions. 5 How do you find the energy change in a reaction. Heat energy is absorbed from the pan to cook the egg.
During this process. Examples of exothermic reactions are. Explain the relationship between ΔH for a reaction and the amounts of reactants and products that undergo reaction.
An exothermic reaction releases energy into the environment. Includes all steps of the scientific method along with a full rubric for. Hydrocarbon combustion neutralization reaction.
1 What are the two energy changes in chemical reactions. Upload your graph and explain the difference between the two reactions using proper chemistry termsCreate a graph of the potential energy for both exothermic and endothermic reactions. In this lab students explore the difference between an endothermic and exothermic reaction through by combining hydrogen peroxide yeast part 1 and baking soda vinegar part 2.
The main difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding whereas exothermic reactions release energy to the surrounding. Download and print the following to use with your Exothermic and Endothermic Reactions Lab Activity. 6CO2 6 H2O heat --- C6H12O6 6O2.
In endothermic processes reactants possess lower potential energy than the product. An exothermic reaction creates heat whereas a endothermic reaction absorbs heat. Endothermic reaction Exothermic reaction Endothermic reaction is a chemical reaction.
Chemical reactions involving the use of energy at the time of dissociation to create a new chemical bond are known as endothermic reactions whereas exothermic reactions are those chemical reactions in which the energy is evolved or released. By contrast endothermic reactions draw heat energy from their surroundings. There is usually a temperature change.
This causes the temperature of the surrounding environment to decrease. 5 rows Photosynthesis is a popular example of an endothermic chemical reaction. In a system energy can do work.
Internal energy is positive in endothermic reactions. 6 What are energy changes. Exothermic and endothermic reactions.
Energy is the capacity to do work. This is a great lab to show the differences in the release and absorption of heat. An increase in temperature is observed in exothermal reactions.
It can change into other forms such as heat sound light etc. Enthalpy energy reactant products. 5 rows The major difference between endothermic and exothermic reactions as their names suggest.
Get 247 Exothermic and Endothermic Reactions assistance at TutorEye. Is this reaction endothermic or exothermic Explain. 4 What is difference between exothermic and endothermic reaction.
3 What are some ways that energy changes occur. Our experts will help you understand the concepts formulas to apply and solve homework problems for. Write an expression for the equilibrium constant for this reaction.
Thus in order to react they absorb the energy from the environment. 7 Does energy change in a. An endothermic reaction feels cold to the touch.
5-1 Exothermic or Endothermic - Lab Answers. Remember to explain the difference using scientific terms such as. 624 Is the change in enthalpy for a reaction an extensive property.
The key difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding environment whereas exothermic reactions release energy to the surrounding environment. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. An exothermic reaction has a negative ΔH and gives off heat to the surroundings.
Liquid water evaporation to form water vapour sublimation of solid carbon. Exothermic vs Endothermic Reactions Lab Worksheets. An endothermic reaction has a positive ΔH and absorbs heat from the surroundings.
Chemical reactions are categorized as endothermic and exothermic reactions according to the energy transfer between the system and the surrounding. An exothermic reaction feels warm to the touch. 2 Do all chemical reactions result in the same change in energy.
Create a graph of the potential energy for both exothermic and endothermic reactions. The final products are stable in exothermic reactions. A decrease in temperature is observed in endothermic reactions.
621 Explain the difference between exothermic and endothermic reactions. Given that the value of the equilibrium constant is 48. Difference Between Endothermic and Exothermic Reactions.
3 marks 2 Explain how a catalyst changes the rate of a reaction. 2 Cooking an egg. Differences between exothermic reaction and endothermic reaction.
Show your understanding using an energy potential diagram. Would you expect this reaction to be fast or slow Explain Explain the effect on equilibrium of the formation of HI form H2reaction with I2 by. 1 Explain the difference between an EXOTHERMIC reaction and an ENDOTHERMIC reaction.
Give the sign ΔH for each type of reaction. An exothermic reaction has a negative enthalpy difference. The endothermic reactions are when the system takes up the energy in the form of light or heat.
5 rows The main difference between exothermic and endothermic reactions is that an endothermic. This is done in the form of heat.
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples

Gcse Chemistry Exothermic And Endothermic Reactions Edexcel 9 1 Youtube
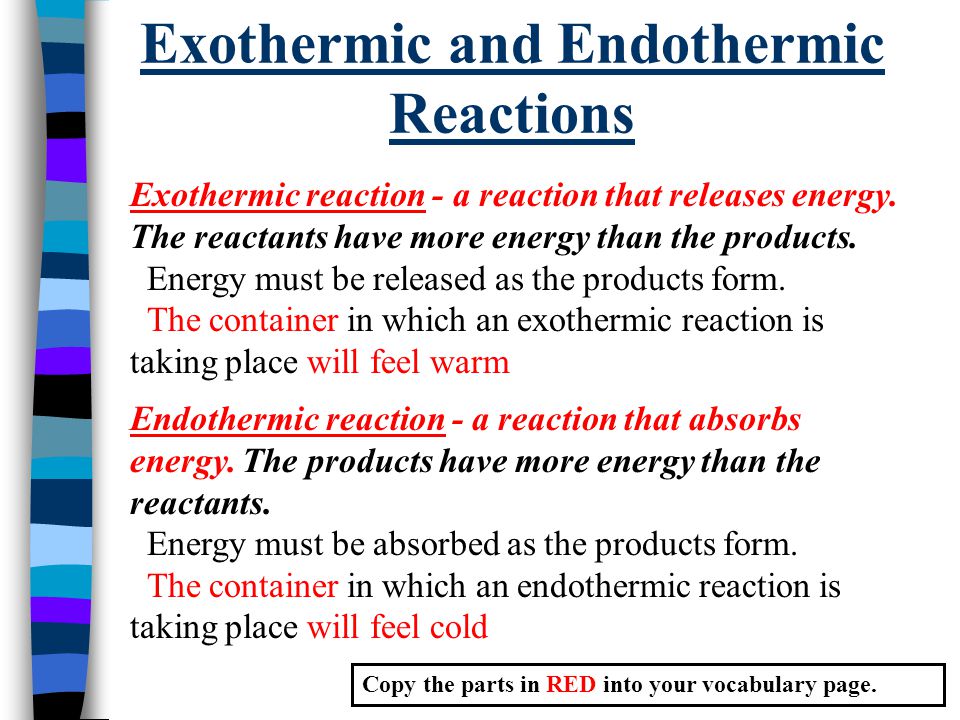
Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up Name 2 Ways You Could Speed Up A Chemical Reaction Ppt Video Online Download
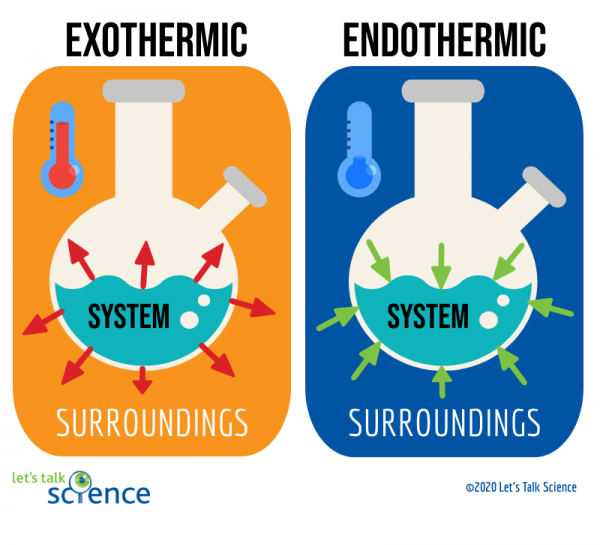
The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science

7 Difference Between Exothermic And Endothermic Reaction With Examples Viva Differences

Exothermic And Endothermic Reactions Labster Theory

What Are Endothermic Reactions With Examples Video
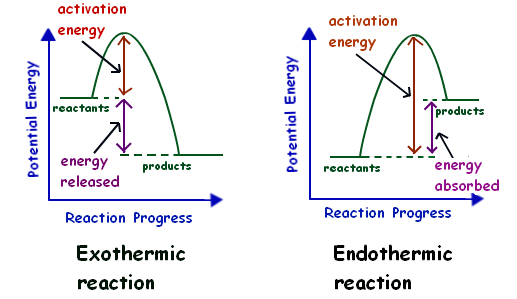
Difference Between Exothermic And Endothermic Reactions Diferr

Endothermic Reaction Definition Equation Graph Examples

Endothermic And Exothermic Reactions Lab Iteachly Com

Exothermic Reactions Release Energy Endothermic Reactions Consume Energy Exothermic Reaction Homeschool Science Chemistry
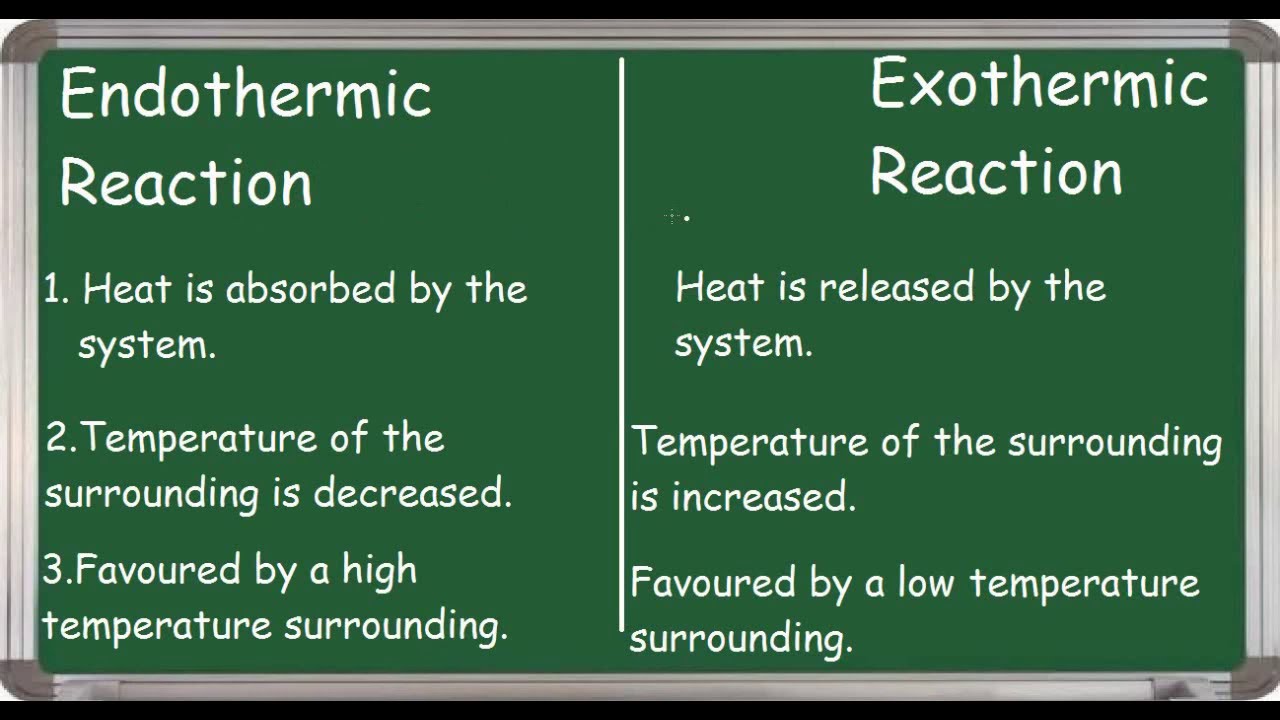
Endothermic Vs Exothermic Reaction Differences Youtube
Endothermic Versus Exothermic Reactions

Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up An Increase In Temperature Makes A Reaction Speed Ppt Download

What Is The Difference Between An Endothermic And An Exothermic Graph Quora
Difference Between Exothermic And Endothermic Difference Between
In An Endothermic Reaction High Temperature Favor High Conversion Why Quora

Endothermic Reaction Definition Equation Graph Examples

What Are Difference Between Exothermic And Endothermic Reactions Definition Types And Importance Chemistry Aesl
Comments
Post a Comment